 Welcome
To The Indian Transplant Registry
Login
Welcome
To The Indian Transplant Registry
Login
Introduction
- Registry – important role in evaluating LT practice and outcome
- Real world data
- Can give immediate and long term picture
- Effectiveness contingent of quality and completeness of data
Advantages
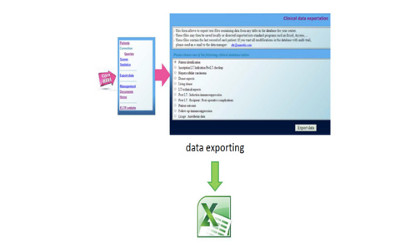
- Capture of data on line
- Queries & corrections
- Statistics (pre-programed + personalized)
- PP presentation and website figures
- Audit trail traceability of all data modification
Key features of ILTR
- Ease of use
- Security
- Anonymity of data
- Reliability and equity of access
- Capture data for recipient and donor characteristics at various intervals in the life cycle of a transplant recipient and living donor :
- Listing time
- Transplant time
- Post-operative
- Discharge time
- First year quarterly follow-up
- Rest 9 years annual follow-up
- Capture immunosuppression regimen, complications and patient reported outcomes
- Application server and database
- Web app for application administration
- Web app for recipient/donor data entry
- Mobile app iOS (iPhone and iPad) data entry and data analytics
- Mobile app Android devices data entry and data analytics
- Web services for data selection/filtering and querying the registry database
- Reports and visual dashboards generation and distribution
- Infrastructure components – user management, session management, audit logs, e-mail component, mobile notification, reminder service, scheduled backup and archival
Levels of engagement – ILTR Lite
- Focus on data gathering with elementary reports/data access
- All centers
- Basic data – demographics, type of LT, outcome
- Ideally all users
- Access to basic demographic and other data published by ILTR
- MOU with centers -agreements to exchange data and cross-check common data
- ? Annual contribution
- The ILTR data will be available to members who’s center is regularly contributing complete data to the ILTR.
- Participating centers will be cited in every publication from the registry
- Data entry screens are simple to start with
- Need to be designed for extensibility to allow for changes in specific data points as the registry matures over time
- Data display screens will be fairly complex
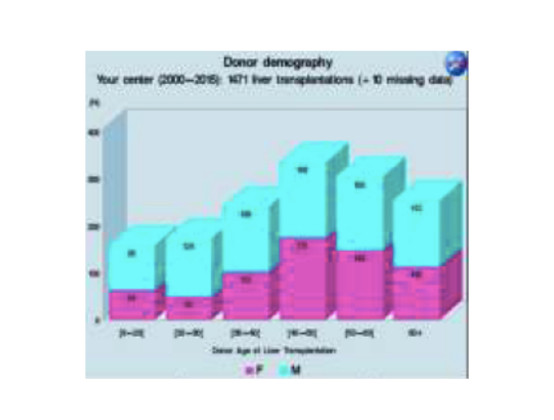
Results published by ILTR